Many clients have been misled to think that Physiokey and Sanakey are the latest SCENAR® devices, made in Germany. The truth is that the former RITM OKB’s distributor in Germany tried to reproduce a year 2000 model RITM SCENAR® and released it on the market in 2015, using the established brand name SCENAR®. The so called Key series devices are not researched clinically and their CE Mark is based on the conventional TENS and SCENAR® trials.
Since then RITM OKB have published numerous materials (links to these publications are below) advising that they are not associated with these products as well as proving that Physiokey and Sanakey technically do not incorporate SCENAR technology.
The latest publication by RITM OKB ZAO explains very well the major difference between the genuine SCENAR and all its analogues – the Transformer. This is the key component that produces the SCENAR signal and which makes the SCENAR® therapy so effective and so unique. The transformers built into the RITM SCENAR® devices are manually adjusted and fine-tuned to the right sensitivity of the human skin and this procedure is the most expensive element of all RITM SCENAR® devices.
All other devices that claim to be SCENAR use cheap mass-production transformers that cannot be adjusted at all. Such an approach simplifies and reduces the products costs, but as result of using this part, the SCENAR signal is deformed and therefore the device is providing little or no therapeutic effect. The image below shows a Physiokey motherboard with a regular, non-adjustable transformer – the part located just above the LCD.
Apart from the transformer, there are numerous other technical problems (which are not as critical as the transformer but very important for the safety and efficacy of the therapy) found in the copy-cat devices such as the lithium battery, energy strength, LCD, electrode port, case material, etc.
The manufacturers of Physiokey devices have stated that these devices can be used for performing techniques of SCENAR therapy.
However, to do so, Physiokey devices should have characteristics similar to SCENAR devices
PHYSIOKEY NOT APPROVED BY FDA
It is important to note that RITM OKB ZAO has 5 full time employed engineers in their Research & Development Department for new devices, who conduct function and firmware research and development. 20 full time employees are responsible for the technical and documentary support, and quality control. Over 100 medical doctors in Russia and overseas are involved in development of new treatment protocols, conduct clinical trials and training. RITM OKB ZAO undergoes two audits per year to maintain the ISO, CE, FDA, TGA Certifications. These are all huge ongoing investments for the development of SCENAR technology that none of the copy-cat device manufacturers invest in their products.
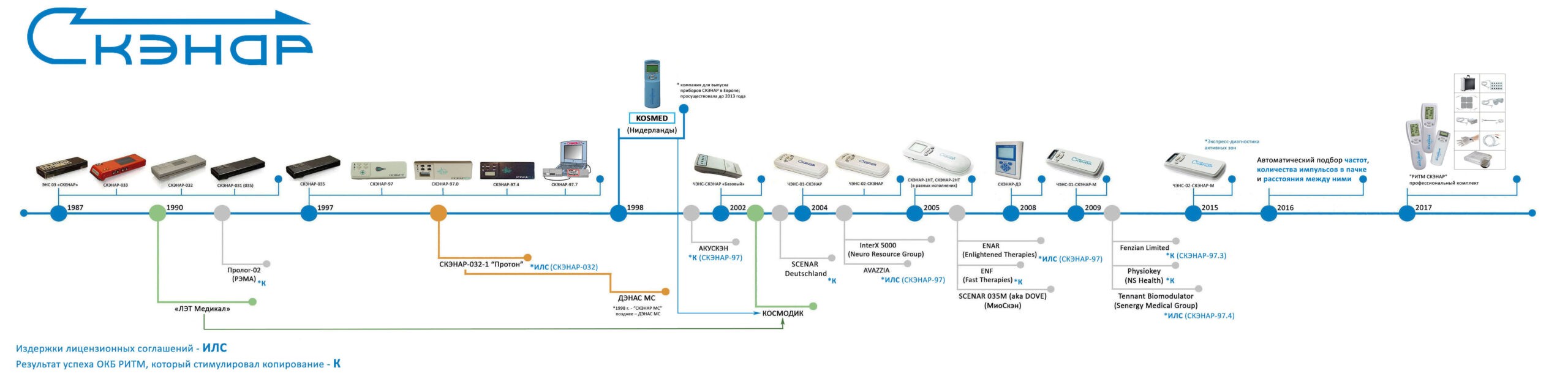
The history of SCENAR® product development is shown in the chart below to provide more information about the Genuine RITM SCENAR® devices and copies